Warnings and precautions
Warnings and precautions
ORSERDU may cause serious side effects
- Increased fat (lipid) levels in your blood (hypercholesterolemia and hypertriglyceridemia). Your healthcare provider will do blood tests to check your lipid levels before and during your treatment with ORSERDU
Before taking ORSERDU, tell your healthcare provider about all your medical conditions, including if you:
- Have liver problems
- Are pregnant or plan to become pregnant. ORSERDU can harm your unborn baby
- Your healthcare provider may do a pregnancy test before you start treatment with ORSERDU
- You should use effective (contraception) birth control during treatment with ORSERDU and for 1 week after the last dose
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ORSERDU
- You should use effective (contraception) birth control during treatment with ORSERDU and for 1 week after the last dose
Females who are able to become pregnant:
Males with female partners who are able to become pregnant:
In the clinical study, most side effects were classified as mild to moderate
Most people did not need to take anti-nausea medication while taking ORSERDU.
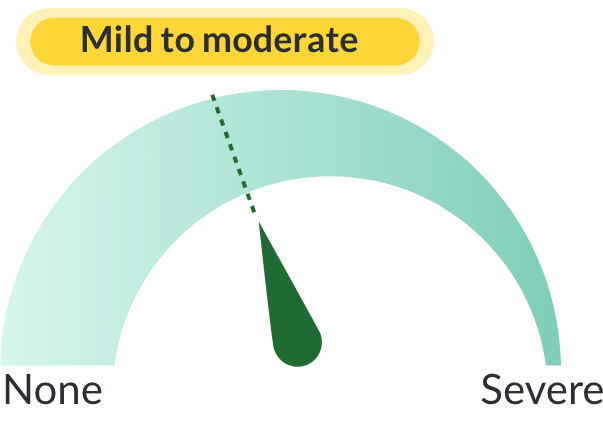

In the clinical study, most side effects were classified as mild to moderate
Most people did not need to take anti-nausea medication while taking ORSERDU.
Of the people in the
clinical study:

needed to reduce treatment with ORSERDU due to side effects

stopped taking ORSERDU due to side effects

had to interrupt their treatment with ORSERDU due to side effects

needed to reduce
treatment
with ORSERDU
due to side effects

stopped taking
ORSERDU due
to side effects

had to interrupt their
treatment with ORSERDU
due to side effects
Let your doctor or healthcare team know if you have any side effects during treatment. Continue taking your medication as prescribed until you speak with your healthcare team. Ask them about tools and strategies that may help manage certain side effects.

IMPORTANT SAFETY INFORMATION AND INDICATION
IMPORTANT SAFETY INFORMATION
ORSERDU may cause serious side effects, including:
- Increased fat (lipid) levels in your blood (hypercholesterolemia and hypertriglyceridemia). Your healthcare provider will do blood tests to check your lipid levels before and during your treatment with ORSERDU
Before taking ORSERDU, tell your healthcare provider about all your medical conditions, including if you:
- Have liver problems
- Are pregnant or plan to become pregnant. ORSERDU can harm your unborn baby
- Your healthcare provider may do a pregnancy test before you start treatment with ORSERDU
- You should use effective (contraception) birth control during treatment with ORSERDU and for 1 week after the last dose
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ORSERDU
- You should use effective (contraception) birth control during treatment with ORSERDU and for 1 week after the last dose
Females who are able to become pregnant:
Males with female partners who are able to become pregnant:
- Are breastfeeding or plan to breastfeed. It is not known if ORSERDU passes into your breast milk. Do not breastfeed during treatment with ORSERDU and for 1 week after the last dose
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. ORSERDU and other medicines may affect each other causing side effects. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
The most common side effects of ORSERDU include:
- Muscle and joint (musculoskeletal) pain
- Nausea
- Increased cholesterol and triglyceride levels in your blood
- Increased liver function tests
- Tiredness
- Decreased red blood cell counts
- Vomiting
- Decreased salt (sodium) levels in your blood
- Increased kidney function test
- Decreased appetite
- Diarrhea
- Headache
- Constipation
- Stomach-area (abdominal) pain
- Hot flush
- Indigestion or heartburn
Your healthcare provider may decrease your dose, temporarily stop, or completely stop treatment with ORSERDU, if you develop certain side effects.
ORSERDU may affect fertility in males and in females who are able to become pregnant. Talk to your healthcare provider if this is a concern for you.
ORSERDU is available as 345 mg and 86 mg tablets.
These are not all the possible side effects of ORSERDU. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
INDICATION
ORSERDU (elacestrant) is a prescription medicine to treat women who have gone through menopause and adult men with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, ESR1-mutated advanced breast cancer or breast cancer that has spread to other parts of the body (metastatic), and whose disease has progressed after endocrine therapy.
Your healthcare provider will perform a test to make sure that ORSERDU is right for you.
It is not known if ORSERDU is safe and effective in children.
Please see Important Facts about ORSERDU.

